Around the world, some coronavirus vaccine candidates have been approved for emergency use. Pfizer/BioNTech, Moderna, and AstraZeneca/Oxford vaccines are used in most Western countries.

The Johnson & Johnson drug recently passed regulatory scrutiny and is now available in several countries.
Furthermore, Russia and China have their own vaccines and have sold their pharmaceuticals to other countries. As of Thursday morning, almost 276 million vaccine doses have been given nationwide, with 58 million patients receiving the entire two-shot regimen.
Related Article: UK Sets to Invest Over $1 Billion for Science
Coronavirus Vaccine

Several recent trials have added to the growing body of evidence that the vaccines successfully reduce serious COVID-19 infections and deaths. The medications are also well-tolerated, with most side effects being minor.
Pain at the injection site and brief flu-like symptoms are among the side effects. Certain vaccines have also been linked to a minimal number of allergic reactions. Doctors are now reporting a strange new COVID-19 vaccine side effect that can take up to a week to manifest in certain patients. Thankfully, it's very minor and shouldn't be a cause for worry.
Follow-Up Shots
After the second shot, new reactions emerge some days later. According to The New York Times, the arm will become raw, sore, itchy, and swollen. The response seems to be benign and should not be mistaken for an illness. According to the letter published in The New England Journal of Medicine, the syndrome was found in patients who received the Moderna vaccine at Massachusetts General Hospital.
Delayed Skin Reactions
The letter describes 12 cases of delayed skin reactions that occurred about 8 days after the initial shot. Approximately six days after the onset, the symptoms vanished. The patients were encouraged to complete the whole course of therapy, and all of them did so.
After the second shot, three patients had a similar reaction. On the other hand, the skin lesions emerged two days after the second jab and continued for up to three days.
Antihistamines were given to some patients, hormones were given to others, and one patient was given an antibiotic by accident.
One of the letter's reporters, Dr. Kimberly Blumenthal, told The New York Times that these signs caused the Massachusetts General Hospital to change the patient handout. "When you have the vaccine, we said it was natural to get redness, scratching, and swelling," she explained.
"We changed the wording to mean it will start seven to ten days after you get the vaccine," says the author. The allergy specialist also expressed her confusion about why the delayed skin reactions only occurred in patients who received the Moderna vaccine rather than the BioNTech medication. Both vaccines are made up of mRNA.
Delayed Reaction
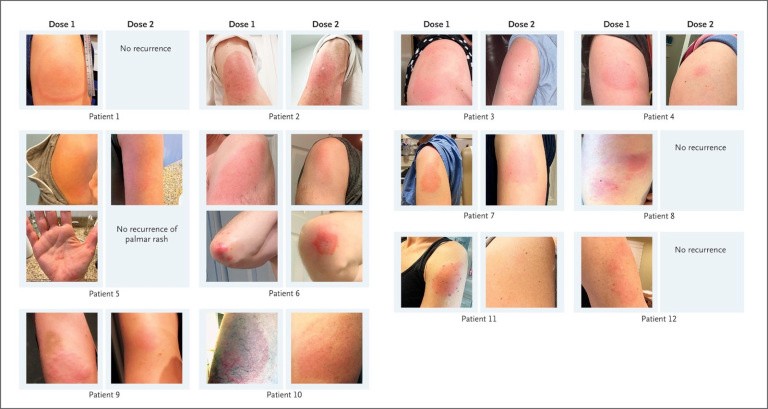
A register has been established at Massachusetts General Hospital to monitor patients who have had delayed skin reactions. So far, Blumenthal has seen over 30 cases, the majority of which are women.
During the Phase 3 experiment, Moderna reported that 0.8 percent of participants had delayed skin reactions after the first injection and 0.2 percent after the second jab. The Moderna trial did not thoroughly describe these delayed reactions, according to the NEJM letter. According to the FDA, the following are the most frequent side effects of the Moderna vaccine:
The pressure at the injection site, tiredness, headache, muscle pain, chills, knee pain, swelling lymph nodes of the same arm as the injection, nausea, and vomiting, and fever were the most frequently recorded side effects, which usually lasted several days.
It's worth noting that these side effects were more common after the second dose than after the first, so vaccine providers and recipients should anticipate any side effects after either dose, but particularly after the second.
ALSO RELATED : Scientists Double Effort to Find Possible Next Pandemic, Caused by Other Zoonotic Diseases
For more health & medicine news, don't forget to follow Nature World News!
© 2025 NatureWorldNews.com All rights reserved. Do not reproduce without permission.





